Catalysts are substances that speed up reactions by
providing an alternative pathway for the breaking and making of bonds. Key to this alternative pathway is a lower
activation energy than that required for the uncatalysed reaction.
Catalysts are often specific for one particular
reaction and this is particularly so for enzymes which catalyse biological
reactions, for example in the fermentation of carbohydrates to produce
biofuels.
Much fundamental and applied research is done by
industrial companies and university research laboratories to find out how
catalysts work and to improve their effectiveness. If catalytic activity can be
improved, it may be possible to lower the temperature and/or the pressure at
which the process operates and thus save fuel which is one of the major costs
in a large-scale chemical process. Further, it may be possible to reduce the
amount of reactants that are wasted forming unwanted by-products.
If the catalyst is in the same phase as the reactants,
it is referred to as a homogeneous catalyst. A heterogeneous catalyst on the
other hand is in a different phase to the reactants and products, and is often
favoured in industry, being easily separated from the products, although it is
often less specific and allows side reactions to occur.
| Process | Catalyst | Equation |
|---|---|---|
| Making ammonia | Iron |  |
| Making synthesis gas (carbon monoxide and hydrogen) | Nickel |  |
| Catalytic cracking of gas oil | Zeolite | Produces: a gas (e.g. ethene, propene) a liquid (e.g.petrol) a residue (e.g. fuel oil) |
| Reforming of naphtha | Platinum and rhenium on alumina | 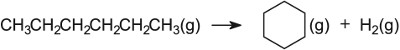 |
| Making epoxyethane | Silver on alumina | 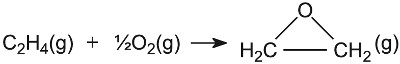 |
| Making sulfuric acid | Vanadium(V) oxide on silica | 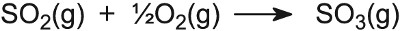 |
| Making nitric acid | Platinum and rhodium |  |
General
requirements for a heterogeneous catalyst
To
be successful the catalyst must allow the reaction to proceed at a suitable
rate under conditions that are economically desirable, at as low a temperature
and pressure as possible. It must also be long lasting. Some reactions lead to
undesirable side products. For example in the cracking of gas oil, carbon is
formed which is deposited on the surface of the catalyst, a zeolite, and leads
to a rapid deterioration of its effectiveness. Many catalysts are prone to
poisoning which occurs when an impurity attaches itself to the surface of the
catalyst and prevents adsorption of the reactants. Minute traces of such a
substance can ruin the process, One example is sulfur dioxide, which poisons
the surface of platinum and palladium. Thus all traces of sulfur compounds must
be removed from the petrol used in cars fitted with catalytic converters.
At
high temperatures, the particles of a finely divided catalyst tend to fuse
together and the powder may 'cake', a process known as sintering. This reduces
the activity of the catalyst and steps must be taken to avoid this. One way is
to add another substance, known as a promoter. When iron is used as the
catalyst in the Haber Process, aluminium oxide is added and acts as a barrier
to the fusion of the metal particles. A second promoter is added, potassium
oxide, that appears to cause the nitrogen atoms to be chemisorbed more readily,
thus accelerating the slowest step in the reaction scheme.
Aluminium
oxide, silicon dioxide, aluminosilicates and zeolites
One
of the most important reactions in which aluminium oxide, Al2O3, (often
referred to as alumina) takes part in an industrial reaction is in platforming,
in which naphtha is reformed over aluminina impregnated with platinum or
rhenium. Both the oxide and the metals have catalytic roles to play, an example
of bifunctional catalysis. There are hydroxyl groups on the surface of alumina
which are, in effect, sites which are negatively charged to which a hydrogen
ion is attached that can act as an acid catalyst.
Silicon
dioxide (silica) is another acidic oxide used in industry. It becomes
particularly active if it has been coated with an acid (such as phosphoric
acid), thereby increasing the number of active acidic sites. For example, the
manufacture of ethanol is achieved by the hydration of ethene using silica,
coated with phosphoric acid:
Aluminosilicates
are also used as catalysts when an acid site is required. These are made from
silicon dioxide (silica) and aluminium oxide. They contain silicate ions,
SiO44- that have a tetrahedral structure which can be linked together in
several ways. When some of the Si atoms are replaced with Al atoms, the result
is an aluminosilicate. Hydrogen ions are again associated with the aluminium
atoms:
Zeolite
catalysts
A
particular class of aluminosilicates that has excited huge interest in recent
years is the zeolites. There are many different zeolites because of the
different ways in which the atoms can be arranged. Their structure of silicate
and aluminate ions can have large vacant spaces in three dimensional structures
that give room for cations such as sodium and calcium and molecules such as
water. The spaces are interconnected and form long channels and pores which are
of different sizes in different zeolites.
A
zeolite which is commonly used in many catalytic reactions is ZSM-5 which is
prepared from sodium aluminate (a solution of aluminium oxide in aqueous sodium
hydroxide) and a colloidal solution of silica, sodium hydroxide, sulfuric acid
and tetrapropylammonium bromide.
It
is, for example, a very effective catalyst for the conversion of methylbenzene
(toluene) to the three dimethylbenzenes (xylenes). Alas, the mixture produced
only contains about 25% 1,4-dimethylbenzene, (p-xylene) the isomer needed for
the manufacture of the polyesters and the rest, 1,2- (o-xylene) and
1,3-dimethylbenzenes (m-xylene), is not wanted in such large quantities.
However,
if the zeolite is washed with phosphoric acid and heated strongly, minute
particles of phosphorus(V) oxide are deposited on the surface making the pores
slightly smaller. This restricts the diffusion of the 1,2- and 1,3-isomers and
they are held in the pores until they are converted into the 1,4-isomer and can
escape (Figure 9).
This
remarkable selectivity enables the yield of the 1,4-isomer to be increased from
25% to 97%.
Figure
9 A zeolite acting an a molecular sieve and a catalyst during the formation of
1,4-dimethylbenzene from methylbenzene.
The
ability of the zeolite to adsorb some molecules and to reject others gives it
the ability to act as a molecular sieve.
For example, in the manufacture of ethanol from ethene or from biomass,
an aqueous solution of ethanol is produced, in which there is 4% water still
present even after repeated distillations. Further purification of ethanol requires
the use of a zeolite which absorbs the water preferentially. Table 2 gives
examples of industrial processes involving zeolites.
The
branched alkene molecule is desorbed into the gas phase until it is readsorbed
on to a metal site where it is hydrogenated to form a branched alkane,
2-methylpropane (isobutane), which is then desorbed into the gas phase.
In
the industrial process, naphtha vapour is passed over platinum and rhenium (ca
0.3% each) which are finely dispersed over aluminium oxide.
The
rhenium is thought to play an interesting role. If a sulfur compound is allowed
to pass over the surface of the catalyst, it is preferentially adsorbed by the
rhenium. If sulfur compounds are not removed, reactions occur leading
eventually to the formation of carbon which causes the activity of the catalyst
to be markedly reduced.
Branched
alkanes have a much higher octane rating than straight chain ones. Not only are
the alkanes now branched, but cycloalkanes are also formed and, from them,
aromatic hydrocarbons. All three classes of hydrocarbon have a higher octane
rating than naphtha. Besides aluminium oxide and silicon dioxide, other oxides
are important catalysts. For example, in the Contact Process used to
manufacture sulfuric acid, the catalyst for the oxidation of sulfur dioxide to
sulfur trioxide is vanadium(V) oxide on the surface of silica:
Potassium sulfate is added as a promoter. Its
mode of action is not absolutely clear but it appears to be because its
presence lowers the melting point of the catalyst, and allows it to spread out
as a very thin layer over the entire surface.
Several
important industrial processes are catalysed by mixed metal oxides. The
surfaces contain two or more different metal atoms, O2- ions and -OH groups.
They are particularly useful in the oxidation of hydrocarbons, where selective
oxidation is required. For example, propene can be oxidized to propenal (acrolein)
using a mixture of bismuth(III) and molybdenum(VI) oxides.
Without
the catalyst, propene is oxidized to a large number of organic compounds,
including methanal and ethanal, and eventually forming carbon dioxide. The
oxygen atoms on the surface of molydenum(VI) oxide are not very reactive,
reacting selectively with propene and breaking the weakest bond in the alkene
to form an allyl radical:
The
allyl radical is then oxidized on the surface to yield propenal. It is
postulated that the allyl radical is oxidized by an oxygen atom that is
adsorbed at a molybdenum site. Another oxygen atom, adsorbed on a bismuth site,
is then transported to the reduced molybdenum site to replace that oxygen.
There is a compensating transport of electrons to complete the cycle.
In
the mechanism for this reaction a hydrogen ion is added at the start, and lost
at the end. The hydrogen ion functions as a catalyst.
Two
other examples are concerned with the production of 2,2,4-trimethylpentane from
2-methylpropene, again using an acid as the catalyst. One uses 2-methylpropane
(Table 3) which yields the alkane directly. The other uses only
2-methylpropene.
The
mechanism of the reaction also involves the addition of a hydrogen ion to a
reactant (Figure 13).
The
alkene is then hydrogenated, using nickel as the catalyst, to 2,2,4-trimethylpentane
(isooctane):
2,2,4-trimethylpentane
is often added to petrol to enhance its anti-knock properties, now that methyl
t-butyl ether (MTBE) is being phased out.
Catalysts
for polymerization reactions
Ziegler-Natta
catalysts
Ziegler-Natta
catalysts are organometallic compounds which act as catalysts for the
manufacture of poly(ethene) and poly(propene). For their work on the production
of polyalkenes, Karl Ziegeler and Giulano Natta were awarded the Nobel Prize in
Chemistry in 1965. The catalysts are prepared from titanium compounds with an
aluminium trialkyl which acts as a promoter:
The
alkyl groups used include ethyl, hexyl and octyl.
The
role of the titanium catalyst can be represented as shown in Figure 14.
The
alkene monomer, for example ethene or propene, attaches itself to an empty
coordination site on the titanium atom and this alkene molecule then inserts
itself into the carbon-titanium bond to extend the alkyl chain. This process
then continues, thereby forming a linear polymer, poly(ethene) or
poly(propene).
The
polymer is precipitated when the catalyst is destroyed on addition of water.
Because it is linear, the polymer molecules are able to pack together closely,
giving the polymer a higher melting point and density than poly(ethene)
produced by radical initiation.
Not
only do Ziegler-Natta catalysts allow for linear polymers to be produced but
they can also give stereochemical control. Propene, for example could
polymerize, even if linear, in three ways, to produce either atactic, isotactic
or syndiotactic poly(propene).
However,
this catalyst only allows the propene to be inserted in one way and isotactic
polypropene is produced.
Even
greater control of the polymerization is obtained using a new class of
catalysts, the metallocenes.
Radical
polymerization
Many
polymers are produced using radical initiators, which act as catalysts (Table
4). For example the polymerization of chloroethene is started by warming it
with a minute trace of a peroxide (R-O-O-R):
In
the case of ethene, by using the free radical process, the resulting polymer
has a lower density and a lower softening point than the poly(ethene) produced
using a Ziegler-Natta catalyst or a metallic oxide. The low density
poly(ethene), LDPE, has side chains because the radicals react not only with
molecules of ethene, by addition, but also with polymer molecules, by a process
known as hydrogen abstraction. The polymer radical can also abstract a hydrogen
atom from its own chain:
Both
of these reactions lead to side chains so that the molecules of the polymer
cannot pack together in a regular way. The polymer thus has a lower melting
point and lower density.
Looking
forward
The
search for catalysts will continue to be one of the highest priorities for the
chemical industry as it seeks to run the processes at as low a temperature and
as near atmospheric pressure as possible, commensurate with a reasonable rate
of reaction.
The
gains from improving catalysts are both financial and environmental, leading to
lower fuel costs, for example the manufacture of methanol and the reduction of
harmful waste gases, for example the manufacture of ethanoic acid. Similarly,
benzene and propene are converted into cumene in the manufacture of phenol,
using a zeolite catalyst in place of aluminium chloride. This means lower
temperatures and pressures are used and the effluent produced is much cleaner.
Further,
catalysts are sought which will favour one specific reaction over another, thus
again making the process much more economic. There are benefits if a catalyst
can be used rather than another chemical that takes part stoichiometrically in
the reaction and cannot be recovered and reused. For example, aluminium
chloride was used for many years to effect the reaction between benzene and a
long chain alkene in the production of alkylbenzene sulfonates, an active
surfactant in many detergents. The aluminium chloride could not be recycled and
became waste as aluminium hydroxide and oxide. Now a solid zeolite catalyst
with acid groups is used and can be reused time and time again with no waste
products.
Another
similar example is in the manufacture of one of the most important polymers
used to make fabrics, polyamide 6 (sometimes known as nylon 6). In this
process, cyclohexanone is converted into caprolactam via the oxime (produced by
the reaction of the ketone with hydroxylamine hydrogensulfate). The oxime is
isomerised by sulfuric acid to caprolactam, and ammonium sulphate is produced
as a by-product. However, again a zeolite catalyst, with acidic sites, is now
being used to effect the rearrangement. The zeolite is regenerated and saves
the use and subsequent waste of sulfuric acid.

No hay comentarios.:
Publicar un comentario